|
Method 1: The “Classical”
Method
The initial production method was first described by Kistler in 1931 (Hrubesh,
1990). The process starts with a mix of tetraalkoxysilane (Si(OR)4, “R represents an alkyl group having from
1 to 5 carbon atoms”) with water and a catalyst like NH4OH (Nogami, 1997). The concentration of Si(OR)4
influences the final density of the silica gel. The mixture is then left to set in a soak solution to allow the bonds between
the particles in the gel form and strengthen. A few days later the soak solution is removed and the mixture is covered in
ethanol to help evaporate the excess water. The mixture obtained at this step is known as alcogel. The alcogel is then placed
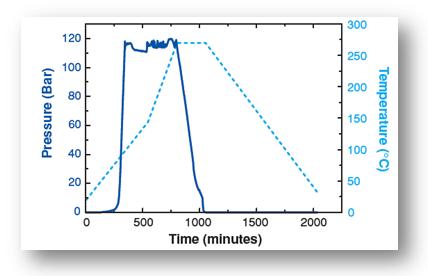
in autoclave that can heat it up to 275°C and a pressure of 120 bars. A very complex heating and pressuring process is then
applied to the alcogel. This process is shown in the figure below. The method takes a total of one week to produce aerogel.
Silica aerogel is usually made from supercritically dried silica, “consisting
of long intertwined chains of small spheres of amorphous silica” (Burchell, 2006). These chains and spheres make up
an extremely low-density rigid three-dimensional system with a high concentration of pores. The production process dictates
the density of the final material (Burchell, 2006).
|
| Temperature and pressure vs. time for the production of silica aerogel |
Method 2: “Teichner’s”
Method
Continued research was performed to find more efficient production methods.
In the 1960’s, a researcher named Stanislaw Teichner at the University of Lyon in France found a less time-consuming
way to produce aerogel. He used tetramethoxysilane (Si(OR3)4), water, and acid or base catalyst (Nogami, 1997). When these ingredients are mixed, ethanol and silicic acid form. The silicic acid particles react with each other
and form small clusters of SiO2. During the formation of silica, water is released. The water reacts with the Si(OR3)4 to form
more silicic acid. This process continues until all the Si(OR3)4 is consumed. The clusters of silica
that form tend to generate spheres which arrange and bond together in random order. Once enough bonds have been established,
a gel has formed. The structure and the density of the final gel are a function of the pH of the initial solution. This new
production method allowed for aerogel to be produced in one day and was the basis for the commercialization of aerogel (Hrubesh,
1990).
Method
3: Making Ultra-Low Density Aerogel
In 1990 a research group at Lawrence Livermore National Laboratory developed a method to produce
ultra-low density aerogels. Their so-called two-step process is similar to the process originally proposed by Kistler in 1931.
Tetramethoxysilane is partially condensed by “limiting the water available to hydrolyze the tetramethoxysilane and is
done under acidic conditions to limit condensation reactions” (Hrubesh, 1990). An important feature of the first step
is to remove all the alcohol and replace it with a non-alcohol solvent. If a high concentration of solvent is used, the final
aerogel will be dense while minimal density is achieved when a minimum amount of solvent is added to the solution. The second
step is the same supercritical extinction process described in Kistler’s method. Using this process, silica aerogels
with densities as low as 0.003g/cm3 have been produced (Hrubesch, 1990).
Cost of Production
Even with the most advanced methods, the production of silica aerogels is still very
costly. This is only partly to blame on the production methods. The raw materials are very costly. 500g of tetramethoxysilane
for example are approximately $200 when purchased in small quantities (Tetramethoxysilane, 2005). Further research is being
done to further improve methods as many possible uses of aerogel only become possible at much lower costs.
|

