|

|
| Aerogel Supporting a Brick |
Aerogels are the lightest and lowest-density solids known to man. “Weighing as little as 3 times that
of air, a single inch thickness of this silica-based material has the internal surface area of a basketball court and can
protect a human hand from the heat of a blowtorch” (Walls, 1998). A piece of aerogel with a density of only 3 milligrams
per cubic centimeter can support up to 4,000 times its own weight as shown in the figure to the right (Silica Aerogels, 2007).

|
| Blue tinted aerogel |
Although aerogels
appear to be transparent, they actually have a slight blue tint as shown in the figure to the left. The Rayleigh scattering
principle predicts that waves will most efficiently be scattered by a scattering center that is about equal to the length
of the wave. In aerogels, the particles themselves are only a few nanometes in size and most pores are about 20nm in diameter.
There are, however, a few scattering centers that scatter visible light. "As scattering efficiency is dependent on the size
of the scattering center, different wavelengths will scatter with varying magnitudes. This causes the reddening of transmitted
light (red light has a longer wavelength, and is scattered less by the fine structure of aerogels) and the blue appearance
of the reflected light off silica aerogels". By reducing the pore size, aerogel
can be made virtually transparent (Science of Silica Aerogels, 2004).
Thermal
Conductivity
|
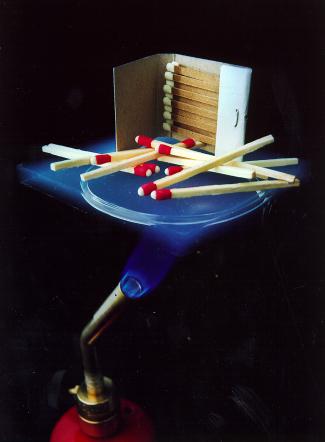
| Matches on Aerogel Over a Flame |
In a material like
aerogel, that does not have free charge carriers available, lattice vibrations are the dominant mode of transporting heat
through the material. This form of transfer is very inefficient in silica aerogels as there is no ordered lattice (this is
also true for refractory SiO2). The pores that are filled with air and make up more than 90% of the volume are
very poor conductors. This leads to low thermal conductivity of aerogel in air and even lower in a vacuum where their pores
do not contribute to conduction at all. The figure to the right shows how insulative even a thin piece of aerogel can
be.
Electrical
Conductivity
Electrical conductivity
in covalently bonded stoichiometric ceramics is through the diffusion of ions through the lattice. The extreme local charges
and the many pores in aerogel make it very difficult for charged species to diffuse through the lattice thus leading to very
low electrical conductivity.
Critical Temperature
The bonds between silicon and oxygen do not break until high temperatures are reached as shown by the very high melting point
of silica. However, the complex and very open three-dimensional structure of silica aerogel starts to collapse at elevated
temperatures. When the structure starts to collapse, the aerogel loses many of its unique properties. The maximum temperature
at which silica aerogel can be used is thus around 500°C.
Click here for the table of properties.
|

