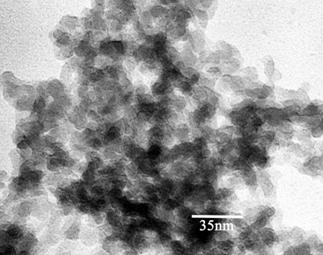
|
| TEM showing the complex silica structure |
Pure stoichiometric silica aerogel has a chemical formula
of SiO2. SiO2 forms a structure in which each silicon atom forms a tetrahedral with oxygen atoms. One
silicon atom is bonded to four oxygen atoms via sp3-hybridization. Each of the resulting SiO4 tetrahedrals has
an effective charge of minus four and shares corners or edges with other tetrahedrals. This gives silica aerogel its
complex three-dimensional structure as shown above. This structure directly influences the properties of aerogel.
|

